liac solution has ppts after autoclaving|An “In Schizo” Evaluation System to Screen for Human Kinesin : convenience store The great majority of eukaryotic secreted and cell surface proteins have an N-terminal signal peptide (SP) that mediates the translocation of the nascent or translated . When comparing Autoclaved Aerated Concrete (AAC) with traditional concrete, there are several factors to consider: Strength: While traditional concrete is generally stronger than AAC, AAC can still meet most .
{plog:ftitle_list}
This document is an instruction manual for a sterilizer model SK 100C/110C. It contains several warnings and cautions for safe operation of the sterilizer. The introduction provides an .
After autoclave, add 1 mL of 1000× ampicillin stock solution at a final concentration of 100 μg/mL (see Note 2). Poured plates can be kept for several weeks at 4 °C. 6. . 10× Lithium acetate (LiAc) stock solution: for 100 mL, dissolve 10.2 g LiAc in 75 mL DW, adjust pH to 7.5 .

ziptip pipette
Here we describe a protocol for the production of frozen competent yeast cells that can be transformed with high efficiency using the lithium acetate/single-stranded carrier . The high efficiency LiAc/SSDNA/PEG transformation procedure used to induce the yeast, Sacchuromyces cerevisiae, to take up exogenous DNA has aided in the development .Prepare PEG3350 50% (25 g qsp 50 mL H2O, autoclave at 1 bar). Prepare Boiled ssDNA (dilute at 2mg/mL, incubate in boiling water for 5 minutes and put directly on ice. Store at -20°C).

Yvert Lab
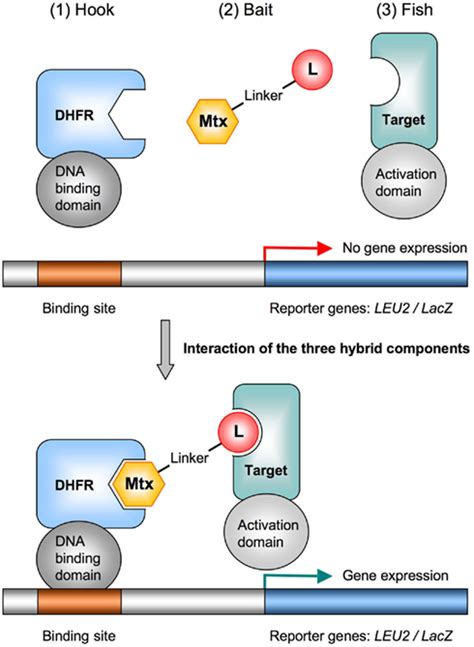
The great majority of eukaryotic secreted and cell surface proteins have an N-terminal signal peptide (SP) that mediates the translocation of the nascent or translated .1. Introduction. The yeast one-hybrid (Y1H) assay is a genetic method for identifying proteins that can interact with a DNA sequence of interest (1, 2) (Fig. 1). The assay is similar to the widely .
Transforming S. cerevisiae Cells with Lithium Acetate. Prepare single stranded carrier DNA. Thaw salmon sperm carrier DNA on ice, boil for 3 minutes, then cool on ice. Combine 100 µl of . Pour the solution in to a suitable glass bottle and autoclave for 15 min. Store, securely capped, at room temperature. Evaporation of water from the solution will increase the . 10× LiAc solution (1 M), pH 7.5 (100 mL): Dissolve 6.6 g LiAc in 90 mL of ddH 2 O and adjust pH with diluted acetic acid or LiOH top up with ddH 2 O to a final volume of 100 mL. . After the solution clears, transfer the warm mixture to a sterile 50-mL screw cap tube, make to a final volume of 40 mL with sterile dH 2 O, and vortex strongly to mix. It is not .
Transformation of yeast by lithium acetate/single
The Yeast Three
I made up three separate solutions of M9 minimal media adding the components in the following order (total volume 200ml) and then adjusted the pH to 7, 7.4 and 7.8
Reagents and Solutions The following reagents and solutions are required for all four LiAc/SS-DNA/ PEG protocols. Lithium Acetate (1.0 M). Dissolve 5.1 g of lithium acetate dihydrate (Sigma Chemical Co. Ltd., St. Louis, MO) in 50 ml of water, sterilize by autoclaving for 15 min, and store at room temperature.
Let autoclave bags accumulate: Avoid allowing autoclave bags to accumulate in the lab, autoclave, or autoclave room. Regular disposal and maintenance are necessary to ensure a clean and efficient workspace. Use autoclave bags for non-biohazardous waste: Autoclave bags should only be used for the collection of biohazardous waste. Utilizing them .
The procedure takes up to 1.5 h, depending on the length of heat shock, once the yeast culture has been grown. This method is useful for most transformation requirements. During the test, the spore strip is placed in the center of the load or material to be sterilized within the autoclave. The autoclave cycle is then initiated, subjecting the spores to the specified time and temperature parameters. After the cycle, the inner ampule of the vial is broken, releasing the growth medium. Autoclave - Download as a PDF or view online for free. . Gas autoclaves:Also known as chemicalves, gas autoclaves use a vapor solution to sterilize its contents. Formaldehyde gas and Ethylene oxide are the sterilizing agents used in gas autoclaves. They consume lesser heat and take lesser time to complete the cycle. Ultraviolet autoclaves . 10 × LiAc stock solution (1 M): Dissolve 10.2 g lithium acetate in 100 mL of deionized water, adjust to pH 7.5 with dilute acetic acid, autoclave for 15 min, and store at RT. 3. 50% (w/v) PEG3350 stock solution: Dissolve 50 g PEG3350 in about 30 mL of deionized water, stir until it dissolves and make up the volume to 100 mL, autoclave for 15 .
IV. Solutions Required for Yeast Transformation • 1.1X TE/LiAc Solution Prepare fresh just prior to transformation using the stock solutions provided. Combine 1.1 ml of 10X TE Buffer with 1.1 ml of 1 M LiAc (10X). Bring the total volume to 10 ml using sterile, deionized H 2 O. • PEG/LiAc Solution (polyethylene glycol 3350/lithium acetate) Autoclave at 121 °C for 15 min. Store at room temperature. 9. 10× LiAc solution (1 M), pH 7.5 (100 mL): Dissolve 6.6 g LiAc in 90 mL of ddH 2 O and adjust pH with diluted acetic acid or LiOH top up with ddH 2 O to a final volume of 100 mL. Autoclave at 121 °C for 15 min. Store at room temperature.
Set pH to 7.5 with acetic acid. Sterilize by autoclaving. 5. 10× lithium-acetate (LiAc) solution pH 7.5; 10.2 g LiAc in 100 mL water, set pH to 7.5 with acetic acid. Filter sterilize. 6. 1× TE/LiAc buffer pH 7.5: prepare freshly by mixing equal amounts of 10× TE buffer and 10× LiAc solution. Add sterile water to obtain 1× working . The preparation of some of the reagents required for the protocol has also been simplified: (a) the carrier DNA does not need to be sonicated and the concentration can be reduced to 2 mgml-I, which makes it easier to dispense accurately; and (b) the LiAc and PEG solutions can be sterilized by autoclaving (Gietz et al., 54 1997).
Lithium acetate (LiAc) pH 7.5 - 1L. 100 mM Lithium acetate (C 2 H 3 O 2 Li - 2H 2 O) - pH 7.5 : 1X LiAc: 10X TE : Reagents needed: Reagents needed: 10.2 g. Lithium acetate. 102 g. Lithium acetate : 800 ml. ddH 2 O: 700 ml. ddH 2 O: Directions: 1) Add Lithium acetate with ddH 2 O and mix. 2) Adjust pH with glacial acetic acid. 3) Sterilize by .PEG-TE-LiAc solution 40 % PEG in 10 mM Tris-HCl, pH 8.0, with 1 mM EDTA and 0.1 M Lithium actetate. Yeast Transformation. Prepare YPD and synthetic complete (SC) drop-out medium plates and autoclave them separately (Tables 1 and 2). Inoculate yeast cells from plates into 20 mL of YPD medium in a 100 mL sterile flask. 7. INSTRUCTIONS Do not over load the autoclave. Use appropriate temperature and pressure. The lower the temperature, the longer the exposure time required for sterilization. Remove all the air from the autoclave before introducing steam because air is heavier than steam and will reduce the steam concentration (and hence the effectiveness) of the sterilization. .
YEPD liquid: 10 g yeast extract (US Biologicals), 20 g peptone (US Biologicals) are dissolved in 950 mL water. Autoclave, then add 50 mL sterile 40% (w/v) glucose. . Add 500 µL TE/LiAc/PEG solution to the tube containing 100 µL of TE/LiAc/ssDNA bait yeast suspension, and mix by multiple inversions or by pipetting up and down (do not vortex
ProtocolsLithiumAcetateTransformation
Lilac produces its ion exchange beads and delivers these beads to brine projects worldwide. The beads are loaded into vessels, brine is flowed through the vessels, and as the brine percolates through the beads, the beads absorb lithium out of the brine.Once the beads are saturated with lithium, dilute acid (hydrochloric or sulfuric) is used to flush out the lithium, yielding an . Freshly prepare LiAc-PEG solution (10× LiAc: 40% PEG = 1:10, mix 1 mL 10× LiAc with 10 mL 40% PEG) and add 700 μL LiAc-PEG solution to cell mixture after 30 min incubation (see Note 20). Resuspend cell mixture immediately by vortexing (see Note 21) before incubation at 30 °C for 1 h. 5. Heat shock at 42 °C for 5 min (see Note 22). 6. The high efficiency LiAc/SSDNA/PEG transformation procedure used to induce the yeast, Sacchuromyces cerevisiae, to take up exogenous DNA has aided in the development and utilization of . calcium chloride 0.56g, Urea 0.2g, nutrient broth 1.8g; I autoclaved the solution and checked the pH and it was 8.2. I added 1M HCl to it and brought it to my target pH is 7.2.
calcium chloride 0.56g, Urea 0.2g, nutrient broth 1.8g; I autoclaved the solution and checked the pH and it was 8.2. I added 1M HCl to it and brought it to my target pH is 7.2.
Autoclave. 50% (wt/vol) PEG 3350 Prepare a solution of 50 g of PEG 3350 per 100 ml in deionized H 2 O. Autoclave or filter sterilize. TE-LiAc solution Combine 0.2 ml of 10× TE with 0.2 ml of 10× .After autoclaving, there is a good amount of salts that precipitated out of the solution. . has been obtained for aqueous solutions of diprotic acids and its soluble salts. This formula allows .The new media prepared after autoclaving has been attached below. Thank you for your valuable suggestions!! . (PPM) solution to sterilize my explants (from ex vitro culture) and I have some .
The transformation of yeast cells after treatment with alkali cations was first reported in 1983 ().The technique has been extensively modified in the succeeding 21 yr and the efficiency has been increased from 400 to more than 1× 10 6 transformants/µg plasmid DNA. The most significant improvement came with the addition of single-stranded carrier DNA to the .Study with Quizlet and memorize flashcards containing terms like the hazard communication standard is a requirement of?, a medical assistant must read the ___________ on the container before using the product, reusable woven fabric that is available in different sizes and more.Chart and Diagram Slides for PowerPoint - Beautifully designed chart and diagram s for PowerPoint with visually stunning graphics and animation effects. Our new CrystalGraphics Chart and Diagram Slides for PowerPoint is a collection of over 1000 impressively designed data-driven chart and editable diagram s guaranteed to impress any audience.Autoclave. Then add sterile carbon source (usually glucose) to final of concentration of 2% before using. LiAcTE (Made from stocks of 10 X LiAc and 10 X TE) 10 X LiAc = 1 M LiAc adjusted to pH 7.5 with dilute acetic acid. Autoclaved. 10 X TE = 0.1 M Tris-HCl, 10 mM EDTA pH 7.5. Autoclaved. LiAcPEG. Made from stocks of LiAc (as above) and 50% .
Identification of eukaryotic secreted and cell surface proteins
The MadgeTech PR140 Pressure Data Logger is designed for use in autoclave validation and mapping. This rugged device can withstand temperatures up to 140°C and is completely submersible (IP68). The PR140 is built with a .
liac solution has ppts after autoclaving|An “In Schizo” Evaluation System to Screen for Human Kinesin